Petroleum Coke (Petcoke): What It Is and How It’s Produced
Petroleum coke, commonly called petcoke, is a carbon-rich solid byproduct of oil refining. It belongs to a group of fuels known as cokes, with petcoke specifically produced through a final cracking stage. This thermo-chemical process breaks long hydrocarbon chains from petroleum into shorter ones inside specialized units called coker units. Unlike metallurgical coke made from coal, petcoke originates from heavy petroleum residues.
Petcoke can also be generated during the production of synthetic crude oil (syncrude) from bitumen in Canada’s oil sands and Venezuela’s Orinoco Belt. In refinery coker units, residual oils from earlier distillation processes are subjected to high heat and pressure. This removes gases and volatile compounds while separating remaining light and heavy oils, leaving behind solid petcoke. These “coking processes,” most commonly delayed coking, are essential refinery operations that transform heavy, low-value residues into a usable, high-carbon fuel and industrial feedstock.
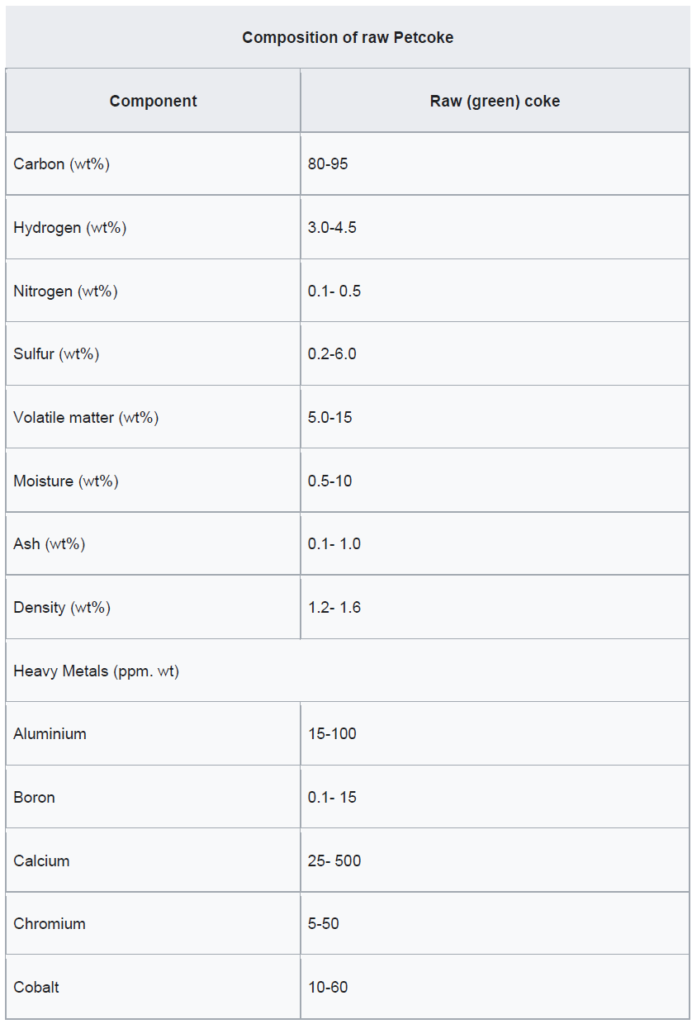
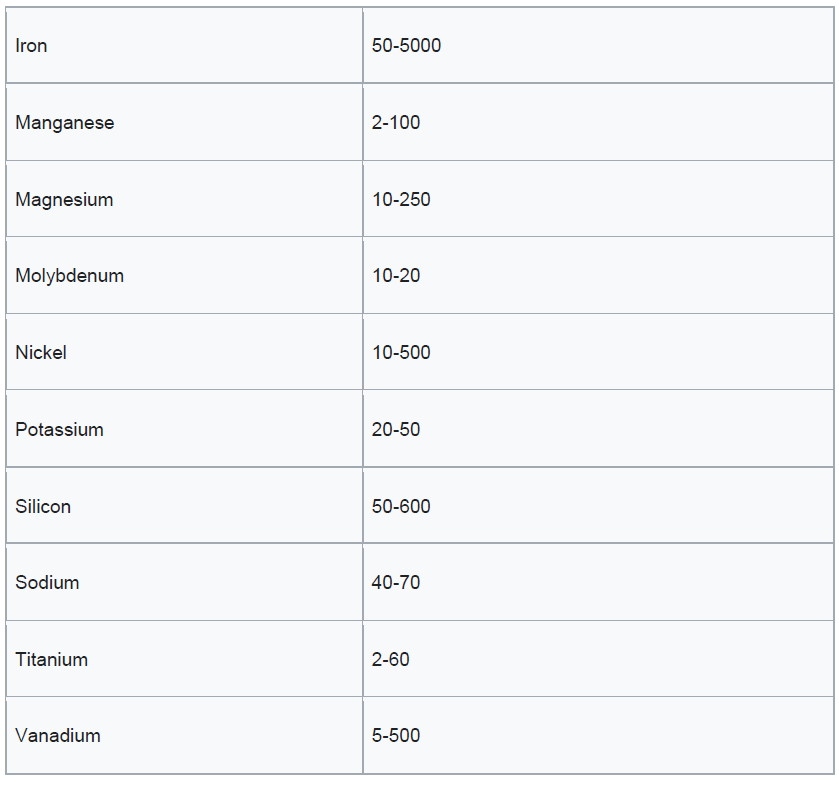
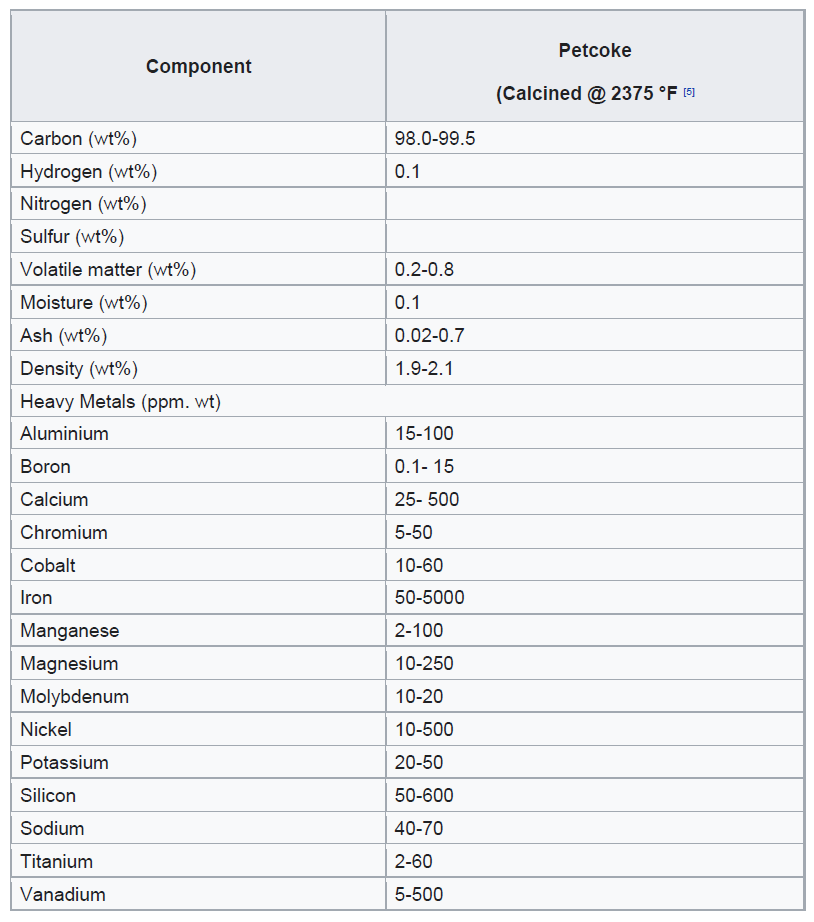
Petroleum coke can be classified into two main grades: fuel grade, which contains higher levels of sulfur and metals, and anode grade, which is lower in these impurities. When petroleum coke is first discharged from the coker unit, it is called green coke—“green” indicating that it is in an unprocessed state. To enhance its quality, green coke undergoes calcination in a rotary kiln. This high-temperature treatment removes residual volatile hydrocarbons, producing calcined petroleum coke. For specialized industrial applications, calcined coke may be further processed in anode baking ovens to create anode coke with precise shapes and physical properties. These anodes play a crucial role in the aluminium smelting process and are also used in the steel industry.
Petcoke is composed of more than 80% carbon and has a significantly higher energy content than coal. However, this also means that when burned, petcoke releases 5–10% more carbon dioxide (CO₂) per unit of energy than coal. On a per-weight basis, its CO₂ emissions can be 30–80% higher. The exact difference depends on factors such as the moisture content of coal—which increases CO₂ output—and the presence of volatile hydrocarbons in both fuels, which can slightly reduce CO₂ emissions per unit of energy.